
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Praesent sapien massa, convallis a pellentesque nec, egestas non nisi. Curabitur non nulla sit amet nisl tempus convallis quis ac lectus. Nulla porttitor accumsan tincidunt. Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia curae; Donec velit neque, auctor sit amet aliquam vel, ullamcorper sit amet ligula.

Understand why tracking sterilized instruments—from decontamination to OR use - is essential for patient safety, accountability, and compliance with global standards.
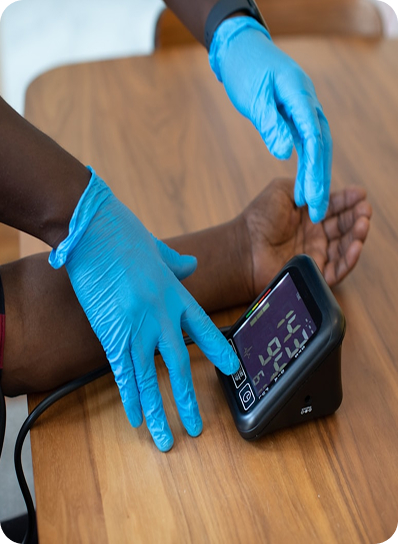
Understand how Ayka’s third-party validated products contribute to regulatory compliance and infection control.

Explore protocols that ensure sterilization efficiency and minimize the risk of surgical site infections.
© Copyright 2025. All Rights Reserved.
Powered by Doors Studio